Prophylactic Cranial Irradiation in Limited-Stage Small Cell Lung Cancer: A Meta-Analysis
By Cheng Chi1, Congcong Wang2, Liangliang Dong2, Xiaoping Li3, Lulu Xu2Affiliations
doi: 10.29271/jcpsp.2024.04.461ABSTRACT
The role of prophylactic cranial irradiation (PCI) in limited-stage small cell lung cancer (LS-SCLC) has been questioned in the era of magnetic resonance imaging (MRI). The purpose of this study was to re-evaluate the efficacy of PCI in patients with LS-SCLC. Three electronic databases were searched, including PubMed, Embase, and the Cochrane Library from January 2012 to April 2022. All relevant publications were included based on the inclusion criteria, and survival data and brain metastasis (BM) rates were extracted and pooled. Ten studies were selected which involved 532 patients who received PCI and 613 patients who did not receive PCI. In pooled estimates, PCI significantly improved overall survival (OS) and progression-free survival (PFS) [hazard ratio (HR) = 0.71, 95% confidence interval (CI): 0.61-0.82, p <0.001; HR = 0.68, 95% CI: 0.48-0.97, p = 0.03, respectively]. Additionally, the use of PCI was associated with a significant reduction in the risk of brain metastasis (BM, risk ratio = 0.64, 95% CI: 0.46–0.90, p = 0.009). In subgroup analyses. The authors found that the PCI effects on OS were independent of region and the use of brain imaging after initial treatment. These findings demonstrate that PCI improves OS and PFS while decreasing the risk of BM in patients with LS-SCLC, implying that PCI remains necessary even in the MRI era.
Key Words: Prophylactic cranial irradiation, Small cell lung cancer, Magnetic resonance imaging, Brain metastasis.
INTRODUCTION
Small cell lung cancer (SCLC) is a deadly neuroendocrine tumour, comprising approximately 13-15% of all lung cancers.1 It is characterised by rapid progression and early metastasis, resulting in a poor prognosis and a median survival of <1 year.2 SCLC frequently metastasizes to the brain, with a 2-year brain metastases (BM) rate of 67% in patients with limited-stage SCLC (LS-SCLC) who achieved a complete response (CR) after treatment. In patients who survive >2 years, the BM rate ranges from 50–80%. The median survival duration for patients with BM is only 4-5 months.3
Most cytotoxic drugs are incapable of penetrating the blood-brain barrier. Since SCLC is extremely responsive to radiotherapy, numerous studies have investigated the use of prophylactic cranial irradiation (PCI) in patients with this cancer. Many studies have indicated that PCI improves survival conditions in patients with LS-SCLC.4 According to a meta-analysis by Auperin et al.5
PCI increased 3-year survival by 5.4% (20.7% vs. 15.3%, p = 0.01) and decreased the cumulative incidence of BM. Based on the findings of this meta-analysis, the American Society of Clinical Oncology recommends that patients who achieve a partial response or CR, subsequent to their initial treatment should be administered PCI. In contrast, a Japanese randomised trial in 2017 found no significant improvement in patients with extensive-stage SCLC (ES-SCLC) without BM confirmed by MRI before PCI.6 Subsequently, the application of PCI in ES-SCLC caused great controversy. As a result, the evaluation of LS-SCLC was considered. Early clinical trials had limitations in tumour staging and BM detection, and PCI was sometimes considered as a treatment for patients with asymptomatic BM. Given the availability of modern staging techniques, such as computed tomography (CT), positron emission tomography, and high-resolution MRI, it raises the question of whether PCI remains necessary. Some studies examined the link between PCI and LS-SCLC. However, the findings have been inconsistent.
Hence, a systematic review and meta-analysis was performed by the authors, employing nearly a decade of clinical studies, to re-evaluate the significance of PCI in patients with LS-SCLC in the era of MRI.
METHODOLOGY
The study protocol was registered in the International Prospective Register of Systematic Reviews database (CRD42022332723, available at https://www.crd.york.ac.uk/prospero/display_record. php?ID=CRD42022332723), and designed following the Cochrane handbook guidelines.7
From January 2012 to April 2022, three electronic databases were searched, namely the Cochrane Library, EMBASE, and PubMed. Various combinations of the following keywords were used without language limitations: small cell lung carcinoma, small cell lung cancer, or SCLC, and prophylactic cranial irradiation or PCI. Additionally, the references of individual studies were manually searched and reviewed articles to guarantee that all pertinent studies were included.
The study’s inclusion criteria were patients who were diagnosed with SCLC through pathology or cytology, and LS disease was confirmed through imaging examinations; brain imaging via MRI or CT was conducted at baseline or after the initial treatment to rule out BM; the intervention of interest was PCI; and the outcome of interest was overall survival (OS) or BM. Only full-text studies were eligible for inclusion. The exclusion criteria were patients diagnosed with non-small cell lung cancer, ES-SCLC, or other metastatic tumours; abstracts, case reports, commentaries, and studies that reported outcomes but did not provide raw data.
As per the aforementioned inclusion and exclusion criteria, two authors independently screened the literature. The data were extracted and recorded using a data extraction form. The Newcastle-Ottawa scale (NOS) (Cache-Ottawa scale) was used to assess the risk of bias in retrospective cohort studies, and the quality of each study was assessed.8 The quality of randomised trials was evaluated using the Cochrane Handbook 5.1 literature quality assessment method.7 Disagreements, if any, were resolved through negotiation.
Stata 15 and Review Manager 5.3 were used for statistical analyses. For a combined estimate, statistical significance was fixed at p <0.05. Two independent reviewers directly extracted and estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for OS, progression-free survival (PFS), and BM risk using other available statistical data based on Tierney’s technique.9 To analyse the statistical heterogeneity of the trial outcomes, the Cochrane Q test and the I2 statistic were employed.10 Results with p <0.1 or I2 >50% were considered heterogeneous, and a random effects model was employed, whereas p >0.1 or I2 <50% showed no significant heterogeneity, and a fixed effect model was used.10 To study the origins of heterogeneity, a subgroup analysis was conducted based on the region and used brain imaging following the initial therapy. In analyses with significant heterogeneity in the overall effect estimates, sensitivity analyses were also conducted to investigate the effects of specific studies.11 To visually assess the potential for publication bias, funnel plots were generated.
RESULTS
A total of 1,797 relevant studies were identified, which were reduced to 1,148 after eliminating duplicates. Following a review of their titles and abstracts, 1,130 studies were subsequently excluded. Three reviews, four studies with missing data, and one study that did not report the outcomes of interest after reviewing the full texts of the remaining 18 articles were excluded. Finally, the meta-analysis included 10 retrospective cohort studies.12-21
The retrospective cohort studies included in the meta-analysis were published between 2012 and 2022 and are listed in Table I along with their basic characteristics.12-21 The total number of patients included was 1,138, with 525 receiving PCI and 613 not receiving it. Brain imaging using MRI or CT was performed at baseline or after the initial treatment to rule out BM. If provided in the literature, propensity score-matched data was used; six studies provided such data.13,15-18,20 For the remaining four articles,12,14,19,20 overall cohort data was used. Multivariate analysis results were preferred over univariate analysis results if provided, as they minimised the influence of confounding factors. In seven studies,14-20 provided HRs and related 95% CIs were obtained, while for the remaining three studies,12,13,21 the HRs were obtained using the method provided by Tierney.9
Table I: Baseline characteristics of included studies in the systematic review and meta-analysis.|
Study |
Year |
Patients, n |
Age, years (Median) |
Male, % |
Median follow-up, mo |
PCI schedule (Gy/fraction) |
Chemotherapy regimen |
Screening for Baseline Brain Metastases, % |
brain imaging after initial treatment, % |
Outcomes |
||||
|
PCI |
No PCI |
PCI |
No PCI |
PCI |
No PCI |
PCI |
No PCI |
|||||||
|
Mads |
2022 |
28 |
21 |
62.2 |
65.5 |
43 |
52 |
33 (9-64) |
26 (3-73) |
25Gy/10f |
EP/EC |
MRI (100) |
MRI was used for patients with neurological symptoms |
probability of developing symptomatic BM, OS, and PFS |
|
Yu |
2022 |
26 |
81 |
60 |
69 |
85 |
85 |
48 |
25-35Gy/10-17f |
platinum-based chemotherapy or others |
MRI (100) |
NR |
OS |
|
|
Yuichi |
2015 |
29 |
95 |
65 |
74 |
93 |
90 |
20 (6-82) |
20 (6-94) |
NR |
UK |
CT/MRI (total:100) |
MRI (69) |
OS, the cumulative incidence of BM |
|
Nobuaki |
2018 |
60 |
20 |
64 |
72.5 |
72 |
55 |
41 (7-151) |
36 (14-145) |
25Gy/10f |
platinum-based chemotherapy |
NR |
MRI (100) |
OS, PFS, the cumulative incidence of BM |
|
Ghanta |
2021 |
83 |
83 |
65.8 |
67.7 |
38.6 |
37.3 |
21.3 (11.5-39.4) |
16.3 (7.9-27.7) |
25-36Gy/10-18f |
platinum-based chemotherapy |
CT/MRI (total:100) |
CT/MRI (total:100) |
OS, neurological survival,BMFS,SBMFS |
|
Qi |
2022 |
75 |
75 |
NA |
NA |
70.7 |
78.7 |
25 (4-102) |
25Gy/10f |
EP/EC |
MRI (100) |
MRI (100) |
OS, PFS |
|
|
Li |
2021 |
69 |
69 |
NA |
NA |
72.5 |
73.9 |
17.8 (8.1-56.7) |
25Gy/10f |
EP |
NR |
MRI (100) |
OS, BMFS |
|
|
Michael |
2019 |
39 |
53 |
NA |
NA |
51 |
53 |
56.7(38-69.4) |
25Gy/10f |
EP/EC or others |
CT (6.5)/MRI(93.5) |
CT/MRI (total:52.2) |
OS, PFS, BMFS |
|
|
Todd |
2020 |
84 |
84 |
65 |
67.5 |
58.3 |
56 |
83.64 (2.5-235) |
83.97 (2.5-235) |
25-30Gy/10-15f |
EP or others |
MRI (100) |
NR |
OS, the cumulative incidence of BM |
|
Yuko |
2021 |
32 |
32 |
NA |
NA |
87.5 |
78.1 |
49.9(7.5-107.5) |
20.6(7.3-168.4) |
25Gy/10f |
NR |
MRI (100) |
CT/MRI (total:65.6) |
OS, BMFS |
|
n: Number; y: Year; mo: Month; PCI: Prophylactic cranial irradiation; MRI: Magnetic resonance imaging; CT: Computed tomography; EP: Etoposide and cisplatin; EC: Etoposide and carboplatin; NA: Not available; NR: not reported; UK: Unknown. BM: Brain metastases; OS: Overall survival; PFS: Progression-free survival; BMFS: Brain metastases-free survival. |
||||||||||||||
|
|
Selection of exposed and non-exposed cohorts |
Comparability |
Outcome of interest |
Overall Quality |
|||||
|
Representativeness of exposed cohort |
Comparability of cohorts |
Ascertainment of exposure |
Outcome present at the start of the study |
Comparability of cohorts |
Assessment of outcome |
Length of follow-up |
Adequacy of follow-up |
||
|
Yuichi 2015 |
* |
* |
* |
* |
|
* |
* |
|
Low |
|
Nobuaki 2018 |
* |
* |
* |
* |
** |
* |
* |
|
High |
|
Michael 2019 |
* |
* |
* |
* |
* |
* |
* |
|
High |
|
Todd 2020 |
* |
* |
* |
* |
** |
* |
* |
|
High |
|
Ghanta 2021 |
* |
* |
* |
* |
** |
* |
* |
|
High |
|
Li 2021 |
* |
* |
* |
* |
** |
* |
* |
|
High |
|
Yu 2022 |
* |
* |
* |
* |
* |
* |
* |
|
High |
|
Mads 2022 |
* |
* |
* |
* |
|
* |
* |
|
Low |
|
Qi 2022 |
* |
* |
* |
* |
** |
* |
* |
|
High |
|
Yuko 2021 |
* |
* |
* |
* |
** |
* |
* |
|
High |
|
Studies that received seven stars at least were judged to be of “high” quality. |
|||||||||
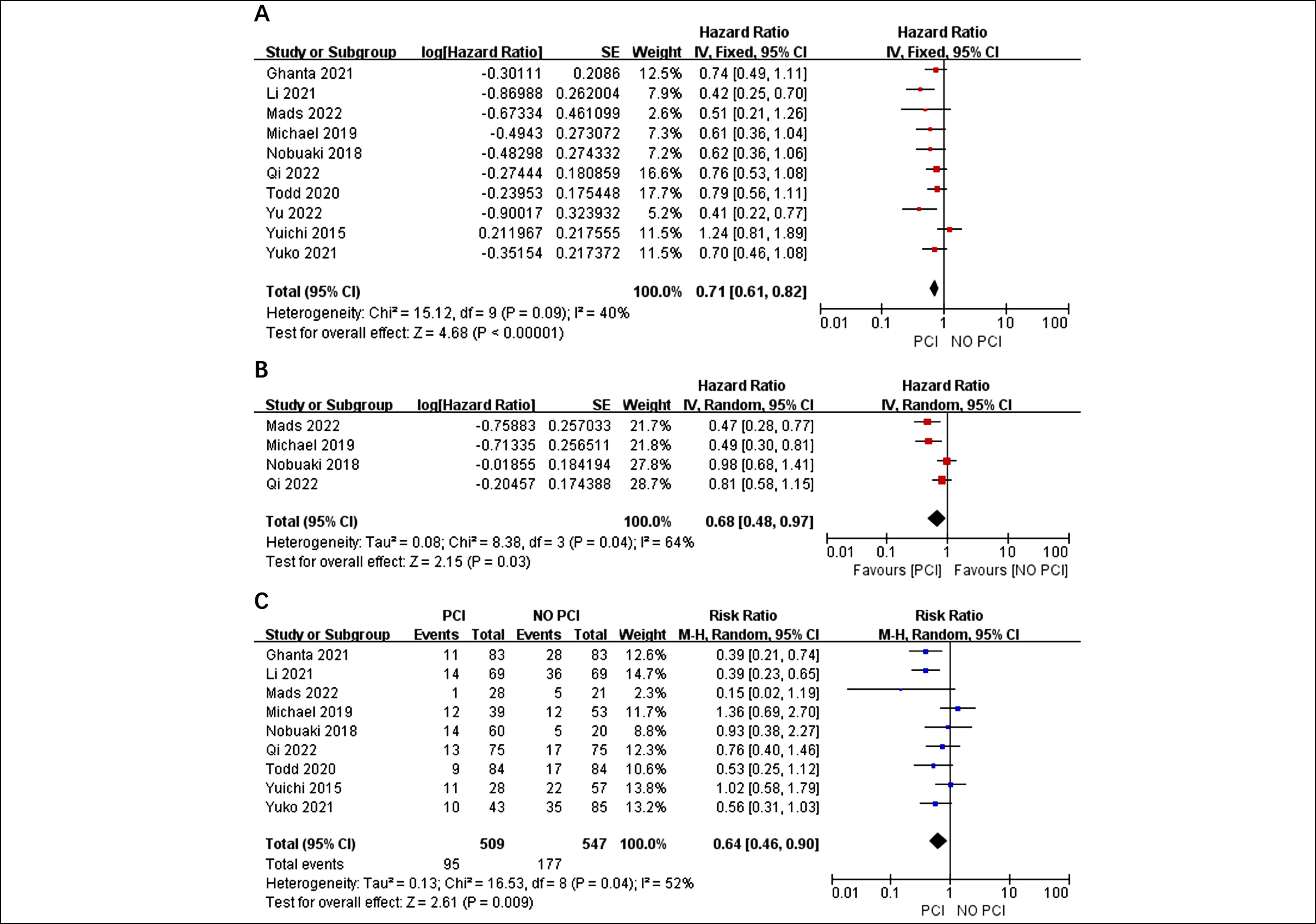 Figure 1: Forest plots for OS (A) PFS (B) and the risk of BM (C) in-patients who received PCI or not.
Figure 1: Forest plots for OS (A) PFS (B) and the risk of BM (C) in-patients who received PCI or not.
All trials included in this analysis were retrospective cohort studies, and their quality using the NOS method were evaluated.8 Of the 10 articles, two were rated as low quality, mainly due to the lack of matching for important confounding factors such as age and incomplete follow-up. Table II presents the quality assessment results for the retrospective cohort studies.
Based on the I2 value of 40%, fixed effect model, findings demonstrated that the use of PCI was related to a significant improvement in OS compared to the control among patients with an HR of 0.71 and a 95% CI of 0.61-0.82 (p<0.001, Figure 2A). Four studies were used to extract HRs,12,14,18,20 and significant statistical heterogeneity was observed (I2 = 64%, p = 0.04). Therefore, a random effect model was used to combine the results. The pooled estimates revealed that the PFS of patients treated with PCI was substantially better than that of the control group (HR=0.68, 95% CI: 0.48-0.97, p=0.03, Figure 2B). The incidence of BM was less prevalent in the PCI group than in the non-PCI group (RR=0.64, 95% CI: 0.46-0.90, p=0.04, Figure 2C). Given the I2 value of 52%, a random effects model was employed.
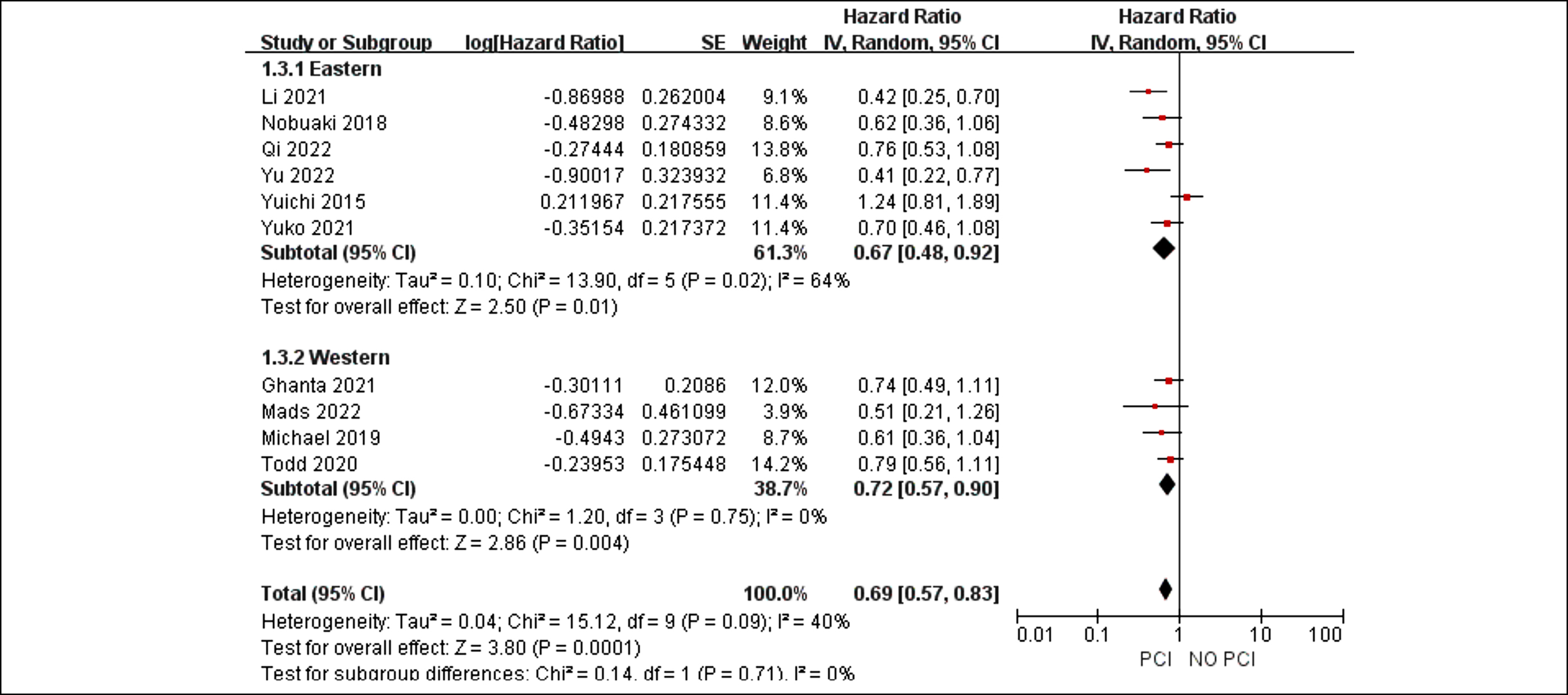 Figure 2: Forest plots for subgroup analysis of region in patients who received PCI or not.
Figure 2: Forest plots for subgroup analysis of region in patients who received PCI or not.
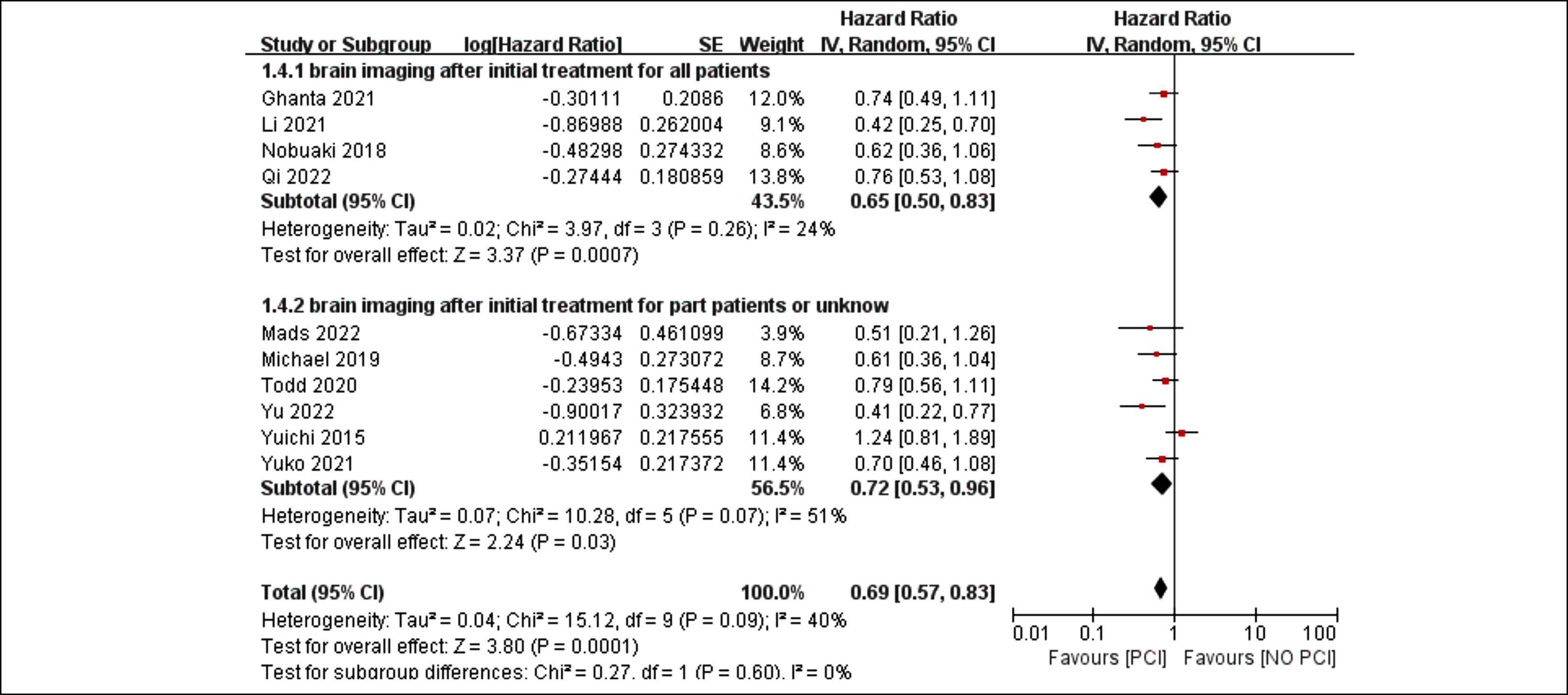 Figure 3: Forest plots for subgroup analysis of the use of brain imaging after initial treatment in patients who received PCI or not.
Figure 3: Forest plots for subgroup analysis of the use of brain imaging after initial treatment in patients who received PCI or not.
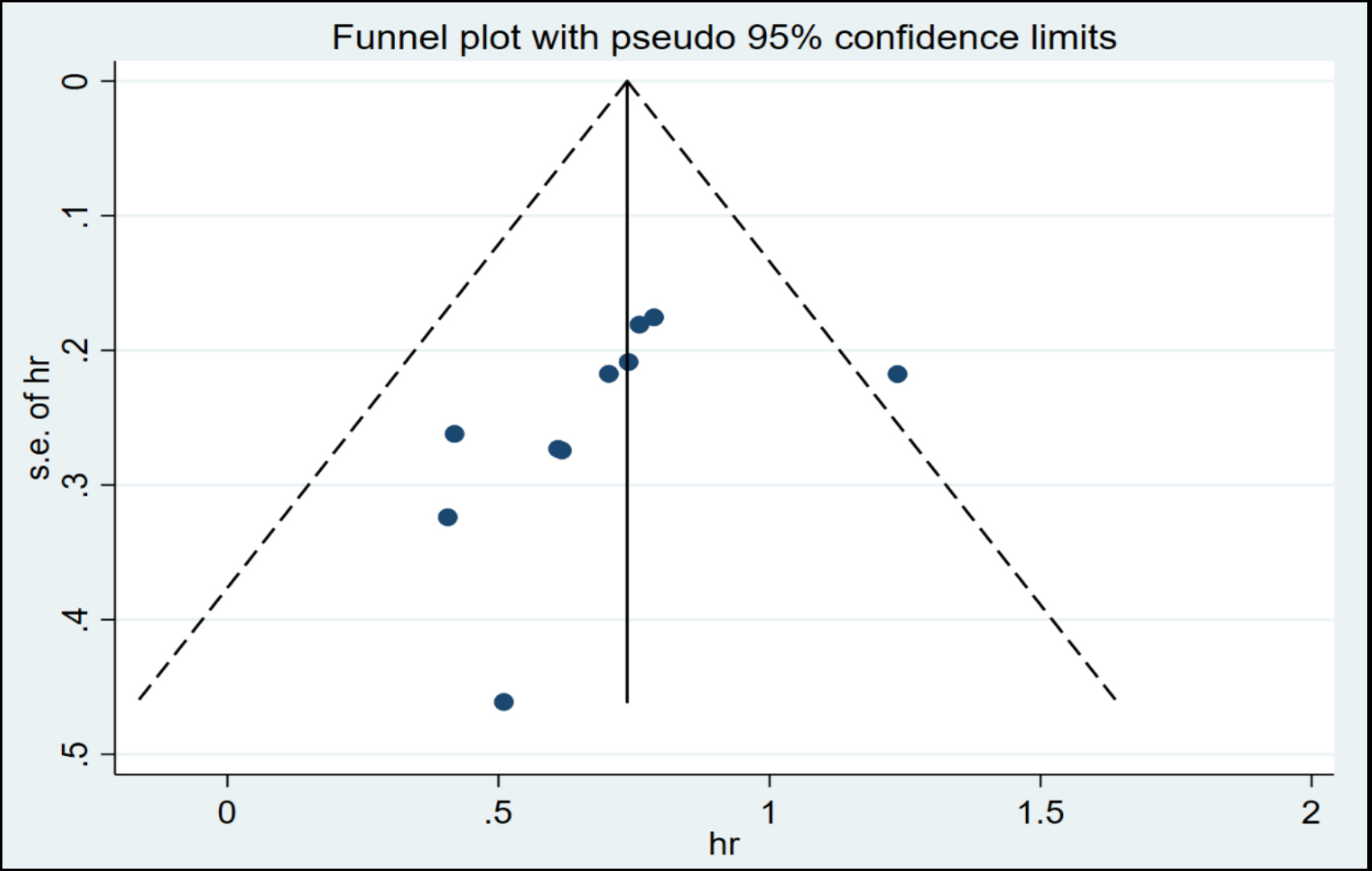 Figure 4: Funnel plot for OS.
Figure 4: Funnel plot for OS.
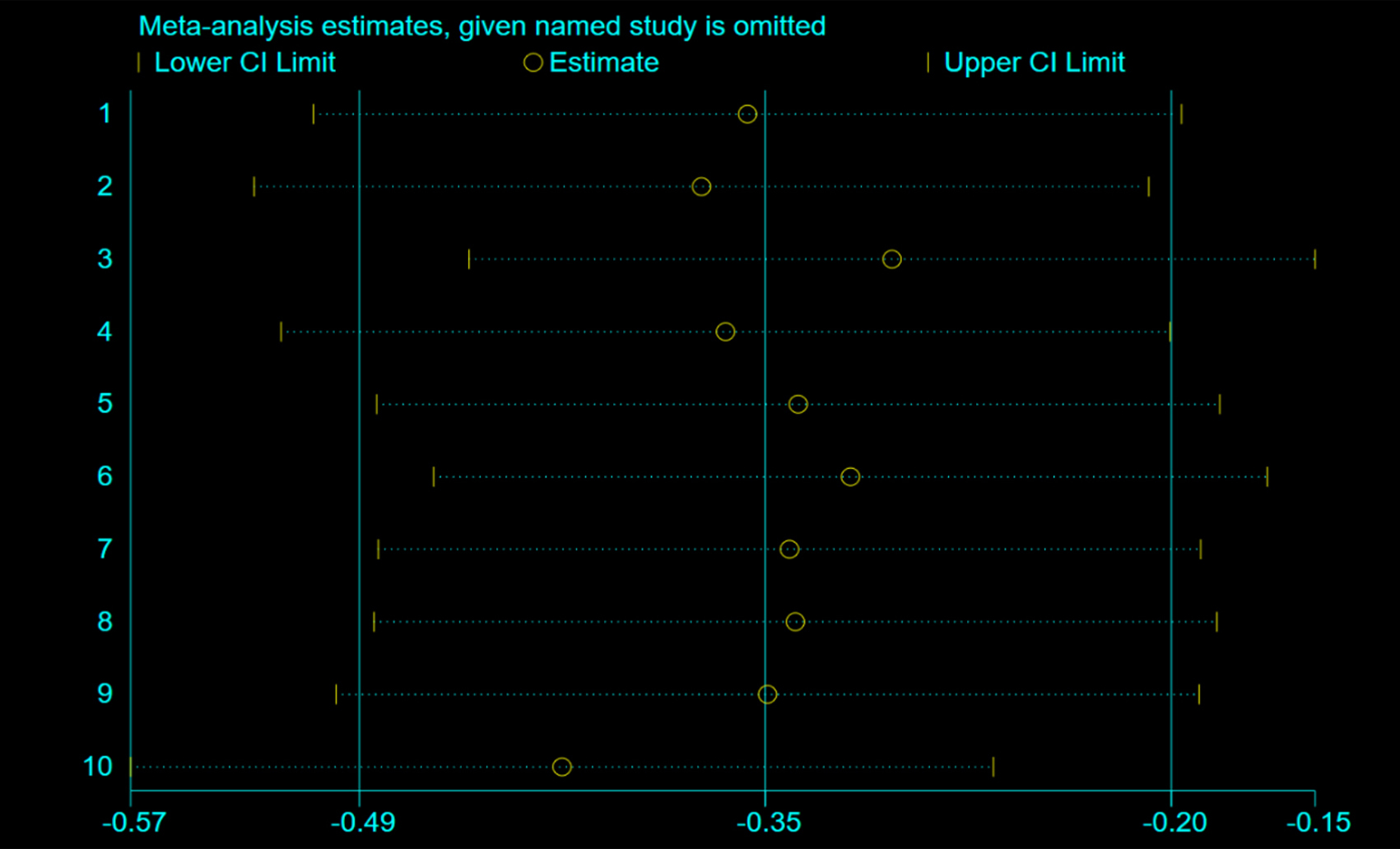 Figure 5: Sensitivity analysis of PCI vs. control for OS in LS-SCLC.
Figure 5: Sensitivity analysis of PCI vs. control for OS in LS-SCLC.
Although the I2 value of 40% in pooled HRs of PCI vs. the control for OS suggests low heterogeneity, further analysis revealed moderate heterogeneity. The findings of the subgroup analyses showed that the treatment effects were consistent and that there were no statistically significant differences in the treatment effects across these subgroups (region, p = 0.71; use of brain imaging following initial treatment, p = 0.6, Figures 3 and 4).
Based on the analysis of funnel plots (Figure 5), there was no indication of publication bias. A sensitivity analysis was conducted by computing the pooled HRs and omitting one study at a time to determine the effect of each study on the overall meta-analysis estimate. The results, presented in Figure 5, support the robustness of the findings.
DISCUSSION
PCI has been the conventional treatment scheme for LS-SCLC since the 1900s. However, a recent prospective study by Takahashi et al. challenged this assumption by demonstrating that PCI did not increase OS (HR=1.27, 95% CI: 0.96-1.68, p=0.094) in patients with ES-SLCL without BM who underwent close brain MRI monitoring and early radiotherapy remediation.6 This study raised questions about the function of PCI in the treatment of ES-SCLC, prompting a re-evaluation of that role. In light of these advancements, this study was conducted to ascertain whether the function of PCI in LS-SCLC has changed. This is the first meta-analysis to investigate the significance of PCI in LS-SCLC patients in the era of MRI. Consistent with the findings of another meta-analysis.5 The present findings imply that PCI exhibited a positive effect on increasing survival and decreasing the incidence of BM in LS-SCLC. However, the Auperin meta-analysis might no longer be applicable in 2022 due to changes in the staging of LS-SCLC. Most studies in the Auperin meta-analysis used CT imaging rather than MRI to assess the involvement of the central nervous system (CNS). Seute et al. compared the occurrence of BM in 481 patients with SCLC across different periods and found that the detection rate of BM was 24% in the MRI era and 10% in the CT era.22 All diagnosed cases of BM in the CT era were symptomatic, whereas 11% of cases in the MRI era were asymptomatic, and multiple BM were detected more frequently. These findings indicate that MRI is superior to CT for detecting CNS disease. In the era of CT, SCLC staging at the initial diagnosis might have been inaccurate, and brain imaging completion to check for BM before PCI treatment was not emphasised. As a result, some patients may have developed asymptomatic BM after enrolling in the PCI trial group, raising the possibility that the effect of PCI in the meta-analysis was obtained from treating a disease that is now more clearly recognised and treated with whole-brain radiotherapy. Only these studies were included where brain imaging was performed at baseline or before PCI, with MRI being the primary method for detecting BM to address this issue. After the first treatment, patients with and without brain imaging underwent subgroup analysis, but no remarkable variations were found between the two groups. The PCI group showed superiority over the control group, indicating that PCI is still necessary for LS-SCLC even after excluding BM in the MRI era. In the authors’ opinion, the aforementioned hypothesis is invalid.
It should be highlighted that administering PCI to patients with LS-SCLC entails higher costs, inconveniences, and a possible risk of toxicity, which has raised concerns among patients and physicians. Neurotoxicity, which has been linked to PCI and described in numerous studies, is of special concern. Numerous studies have noted long-term consequences, including severe memory loss, intellectual disability, dementia, and ataxia.23-30 In a notable pooled analysis of the Radiation Therapy Oncology Group (RTOG) 0212 and RTOG 0214 trials, at 6 and 12 months following treatment, PCI was discovered to be linked to an increase in both tested and self-reported cognitive toxicity.25 It is worth noting that developments in radiation delivery methods may be able to lessen PCI toxicity. According to research, administering 25 Gy in 10 once-daily fractions is a feasible approach for PCI in SCLC, and increasing the dose does not diminish the likelihood of developing BM but increases neurotoxicity.4,31 Furthermore, developing approaches like hippocampus sparing and the use of protective medicines like memantine may improve the therapeutic index.32 However, the authors were unable to pool the incidence of toxicity in this meta-analysis due to insufficient data.
The present study has some limitations. First, the sample size was small, and all the studies were retrospective. Retrospective studies are prone to selection bias and selective reporting and are considered less conclusive than randomised controlled trials. However, no prospective studies on the effect of PCI on LS-SCLC have been conducted in the past decade or longer. Second, some studies did not provide direct HRs, which required us to estimate them from the survival curve, potentially leading to inaccuracies. Third, there was statistical heterogeneity in the meta-analysis that could not be explained which might be due to various factors, such as differences in baseline characteristics, including response after initial treatment, the timing of PCI, follow-up time, radiation dose, different tumour and node stages, etc. However, further analysis was not feasible due to the limited data available. Finally, although no obvious publication bias was found, it is possible that some studies were not included, such as unpublished trials or abstracts, due to data unavailability.
With the advent of immunotherapy, a new era has begun. IMPower133 and CASPIAN studies have confirmed that combining immunotherapy with chemotherapy can significantly prolong the OS of patients with ES-SCLC.33,34 Based on the findings of these two studies, the Food and Drug Administration has approved the Programmed death-ligand 1 inhibitors Atezolizuma and Durvalumab in combination with chemotherapy as a first-line treatment for ES-SCLC. In LS-SCLC, although the Small Cell Lung Carcinoma Trial With Nivolumab and IpiliMUmab in Limited Disease study did demonstrate positive outcomes,35 it did identify a trend of OS benefit in the hyperfractionated radiotherapy subgroup. Moreover, immune checkpoint inhibitors have been shown to cross the blood-brain barrier and produce objective responses in patients with known BM.36 Two ongoing clinical trials, the Southwest Oncology Group trial and the Prophylactic Cerebral Irradiation or Active Magnetic Resonance Imaging Surveillance in SCLC patients study are currently underway to gain a better understanding of the role of PCI in the modern staging era of immunotherapy. It remains to be seen whether their results will change the role of PCI.
CONCLUSION
This meta-analysis indicates that PCI might enhance survival and lower the probability of BM in patients with LS-SCLC, even after ruling out BM through MRI or CT before PCI. However, given the limited number of studies included in this meta-analysis, all of which were retrospective cohort studies, additional randomised controlled trials are required to validate these results.
COMPETING INTEREST:
The authors declared no conflict of interest.
AVAILABILITY OF DATA AND MATERIALS:
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
AUTHORS' CONTRIBUTION:
LX, XL: Conceived, designed the research, and critically revised the manuscript.
LX, CW: Screened the literature and conducted the quality rating.
LX, CC, LD: Involved in conducting the statistical analyses.
LX, CW, CC: Drafted the manuscript.
All authors read and approved the final manuscript for publication.
REFERENCES
- Oronsky B, Reid TR, Oronsky A, Carter CA. What's new in SCLC? A review. Neoplasia 2017; 19(10):842-7. doi: 10. 1016/j.neo.2017.07.007.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65(1):5-29. doi: 10.3322/caac.21254.
- Tai P, Assouline A, Joseph K, Stitt L, Yu E. Prophylactic cranial irradiation for patients with limited-stage small-cell lung cancer with response to chemoradiation. Clin Lung Cancer 2013; 14(1):40-4. doi: 10.1016/j.cllc.2012.04.005.
- Le Péchoux C, Dunant A, Senan S, Wolfson A, Quoix E, Faivre-Finn C et al. Prophylactic cranial irradiation (PCI) collaborative group. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): A randomised clinical trial. Lancet Oncol 2009; 10(5):467-74. doi: 10. 1016/S1470-2045(09)70101-9.
- Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. N Engl J Med 1999; 341(7):476-84. doi: 10.1056/NEJM199908123410703.
- Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017; 18(5):663-71. doi: 10.1016/S1470- 2045(17)30230-9.
- Shuster JJ. Review: Cochrane handbook for systematic reviews for interventions, Version 5.1.0, published 3/2011. Julian P.T. Higgins and sally green, editors. Res Synth Methods 2011; 2:126-30. doi:10.1002/jrsm.38.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: The medical education research study quality instrument and the newcastle-ottawa scale-education. Acad Med 2015; 90:1067-76. doi: 10.1097/ACM. 0000000000000786.
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. doi: 10.1186/1745- 6215-8-16.
- Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ 1994; 309(6965):1351-5. doi: 10.1136/bmj.309.6965.1351.
- Copas JB, Shi JQ. A sensitivity analysis for publication bias in systematic reviews. Stat Methods Med Res 2001; 10(4): 251-65. doi: 10.1177/096228020101000402.
- Ozawa Y, Omae M, Fujii M, Matsui T, Kato M, Sagisaka S, et al. Management of brain metastasis with magnetic resonance imaging and stereotactic irradiation attenuated benefits of prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. BMC Cancer 2015; 15:589. doi: 10.1186/s12885-015-1593-2.
- Mamesaya N, Wakuda K, Omae K, Miyawaki E, Kotake M, Fujiwara T, et al. Efficacy of prophylactic cranial irradiation in patients with limited-disease small-cell lung cancer who were confirmed to have no brain metastasis via magnetic resonance imaging after initial chemoradiotherapy. Oncotarget 2018; 9(25):17664-74. doi: 10.18632/oncotarget. 24830.
- Farris MK, Wheless WH, Hughes RT, Soike MH, Masters AH, Helis CA, et al. Limited-stage small cell lung cancer: Is prophylactic cranial irradiation necessary? Pract Radiat Oncol 2019; 9(6):e599-e607. doi: 10.1016/j.prro.2019.06.014.
- Pezzi TA, Fang P, Gjyshi O, Feng L, Liu S, Komaki R, et al. Rates of overall survival and intracranial control in the magnetic resonance imaging era for patients with limited-stage small cell lung cancer with and without prophylactic cranial irradiation. JAMA Netw Open 2020; 3(4):e201929. doi: 10.1001/jamanetworkopen.2020.1929.
- Li J, Ding C, Yang C. Prophylactic cranial irradiation confers favourable prognosis for patients with limited-stage small cell lung cancer in the era of MRI: A propensity score matched analysis. J Med Imaging Radiat Oncol 2021; 65: 778-85. doi: 10.1111/1754-9485.13269.
- Ghanta S, Keller A, Rodriguez-Lopez JL. Utility of prophylactic cranial irradiation for limited stage small cell lung cancer in the modern era with magnetic resonance imaging surveillance. Clin Oncol (R Coll Radiol) 2021; 33:e323-e30. doi: 10.1016/j.clon.2021.03.018.
- Qi C, Li W, Li H. Benefits of prophylactic cranial irradiation in the MRI era for patients with limited stage small cell lung cancer. Front Oncol 2022; 12:833478. doi: 10.3389/fonc.2022.833478.
- Lim YJ, Song C, Kim HJ. Korean Association for lung cancer; Korea central cancer registry. Survival impact of prophylactic cranial irradiation in small-cell lung cancer in the modern era of magnetic resonance imaging staging. Radiat Oncol 2022; 17(1):26. doi: 10.1186/s13014-022-01994-8.
- Held MK, Hansen O, Schytte T, Hansen KH, Bahij R, Nielsen M, et al. Outcomes of prophylactic cranial irradiation in patients with small cell lung cancer in the modern era of baseline magnetic resonance imaging of the brain. Acta Oncol 2022; 61(2):185-92. doi: 10.1080/0284186X.2021.1974553.
- Inoue Y, Tsujino K, Sulaiman NS, Marudai M, Kajihara A, Miyazaki S, et al. Re-evaluation of prophylactic cranial irradiation in limited-stage small cell lung cancer: A propensity score matched analysis. J Radiat Res 2021; 62(5):877-83. doi: 10.1093/jrr/rrab053.
- Seute T, Leffers P, ten Velde GP, Twijnstra A. detection of brain metastases from small cell lung cancer: Consequences of changing imaging techniques (CT versus MRI). Cancer 2008; 112(8):1827-34. doi: 10.1002/cncr.23361.
- Ohonoshi T, Ueoka H, Kawahara S, Kiura K, Kamei H, Hiraki Y, et al. Comparative study of prophylactic cranial irradiation in patients with small cell lung cancer achieving a complete response: A long-term follow-up result. Lung Cancer 1993; 10(1-2):47-54. doi: 10.1016/0169-5002(93)90308-k.
- Arriagada R, Le Chevalier T, Borie F, Rivière A, Chomy P, Monnet I, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 1995; 87(3):183-90. doi: 10.1093/jnci/87.3.183.
- Gregor A, Cull A, Stephens RJ, Kirkpatrick JA, Yarnold JR, Girling DJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer 1997; 33(11):1752-8. doi: 10.1016/s0959-8049(97)00135-4.
- Nakahara Y, Takagi Y, Okuma Y, Hosomi Y, Okamura T, Shibuya M, et al. Neurotoxicity due to prophylactic cranial irradiation for small-cell lung cancer: A retrospective analysis. Mol Clin Oncol 2015; 3(5):1048-52. doi: 10.3892/mco. 2015.581.
- Rule WG, Foster NR, Meyers JP, Ashman JB, Vora SA, Kozelsky TF, et al. Prophylactic cranial irradiation in elderly patients with small cell lung cancer: Findings from a north central cancer treatment group pooled analysis. J Geriatr Oncol 2015; 6(2):119-26. doi: 10.1016/j.jgo.2014.11.002.
- Gondi V, Paulus R, Bruner DW, Meyers CA, Gore EM, Wolfson A, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: Pooled secondary analysis of radiation therapy oncology group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys 2013; 86(4):656-64. doi: 10.1016/j.ijrobp.2013. 02.033.
- Farooqi AS, Holliday EB, Allen PK, Wei X, Cox JD, Komaki R. Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: Do all patients benefit? Radiother Oncol 2017; 122(2):307-12. doi: 10.1016/j.radonc.2016.11.012.
- Slotman BJ, Mauer ME, Bottomley A, Faivre-Finn C, Kramer GW, Rankin EM, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: Short-term health-related quality of life and patient reported symptoms: Results of an international phase III randomized controlled trial by the EORTC radiation oncology and lung cancer groups. J Clin Oncol 2009; 27(1):78-84. doi: 10.1200/JCO. 2008.17.0746.
- Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 81(1):77-84. doi: 10.1016/j.ijrobp.2010.05.013.
- Robin TP, Rusthoven CG. Strategies to preserve cognition in patients with brain metastases: A review. Front Oncol 2018; 8:415. doi: 10.3389/fonc.2018.00415.
- Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. IMpower133 study group. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379(23):2220-29. doi: 10.1056/NEJMoa1809064.
- Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019; 394(10212):1929-39. doi: 10.1016/S0140-6736(19) 32222-6.
- Peters S, Pujol JL, Dafni U, Dómine M, Popat S, Reck M, et al. ETOP/IFCT 4-12 STIMULI collaborators. Consolidation nivolumab and ipilimumab versus observation in limited-disease small-cell lung cancer after chemo-radiotherapy - results from the randomised phase II ETOP/IFCT 4-12 STIMULI trial. Ann Oncol 2022; 33(1):67-79. doi: 10.1016/j. annonc.2021.09.011
- Kamath SD, Kumthekar PU. Immune checkpoint inhibitors for the treatment of central nervous system (CNS) metastatic disease. Front Oncol 2018; 8:414. doi: 10.3389/fonc.2018.00414.